How Aortic Aneurysms Become Aortic Catastrophes
LEARNING Objectives
- Define the three tunics, the three sections of the thoracic aorta, aortic aneurysm, and aortic dissection.
- Explain the process by which an aortic aneurysm becomes a dissection.
- Formulate a series of care plans that appropriately account for the patient’s presentations and aortic dissection’s most common complications.
KEY Terms
Abdominal aorta: The portion of the aorta that’s housed within the abdomen between the diaphragm and iliac bifurcation.
Aortic aneurysm: The chronic process by which inflammation usually causes destruction and weakening on the aorta’s wall, allowing it to expand by at least 50% its expected size.
Aortic dissection: The disease process by which the inner layer of the aorta (tunica intimna) tears and allows blood to flow between the layers of the aorta, causing these layers to dissect from one another.
Thoracic aorta: The portion of the aorta that’s housed between the heart and the diaphragm within the chest cavity.
In prehospital emergency medicine, there are few diseases that carry the same high priority and respect as aortic catastrophes. From very early in our training, providers at all levels are taught to search for, suspect and ultimately fear derangements of the aorta. And in all reality, the respect and attention this illness demands isn’t in vain.
The aorta is the largest blood vessel within the body and originates directly from the heart. It carries all of the body’s circulating blood volume at the point it leaves the left ventricle. When the integrity of the aorta’s wall is compromised, a person can exsanguinate in just a few short contractions of the heart. At the risk of sounding cliché, aortic catastrophes can kill a patient in a heartbeat.
Patients with an acute illness to their aorta are unbelievably fragile, requiring tight control of their condition by educated and dedicated providers. This frailty coupled with the risk of instantaneous and irreversible cardiovascular collapse are what give aortic catastrophes such a reputation and need for such surveillance.
ANATOMY & PHYSIOLOGY
The aorta is the primary arterial vessel within the thorax and abdomen. Due to its prominence within these two compartments, the aorta is most basically classified into two regions: the thoracic aorta (TA) and the abdominal aorta (AA).
Starting in the mid-chest on the anterior surface of the left ventricle, the TA begins its dance through the chest, twisting its way through the other great vessels. Ultimately, the aorta is a candy cane-shaped vessel with the hook wrapping along the top of the heart and the staff running parallel to the spine. (See Figure 1.)
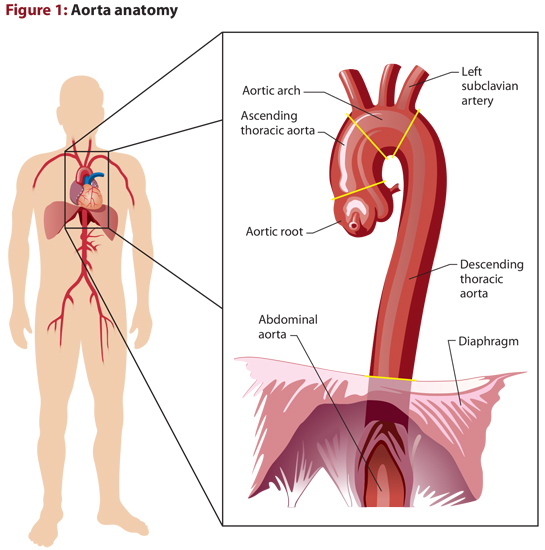
Because of the unique anatomy that the proximal TA has, it’s further categorized based on the direction that blood is traveling within certain portions:
- Ascending aorta: The portion where blood ascends from the root to the point where it begins to obliquely flow across the top of the heart. The most proximal portion of the ascending aorta is known as the aortic root, and is where the right and left coronaries originate.
- Aortic arch: The portion of the TA where blood travels obliquely between the ascending and descending portions. The vessels that supply the arms and head with their circulation originate on the arch, and are important for diagnostics and prognosis as we’ll see later on.
- Descending aorta: The final portion of the TA where blood descends from the arch until it reaches the diaphragm.
As the TA travels inferiorly along the left border of the spine, it dives through an opening within the diaphragm. Once the aorta passes through the diaphragm, it’s referred to as the AA.
The AA continues to travel inferiorly as it provides all of the abdominal organs with their perfusion. Once it gets to the level of L4, it bifurcates into the iliac arteries, which supply the lower extremities with oxygenated blood.
This gross anatomy of the aorta plays a significant role in the symptoms, exam findings, and prognosis of the patients with an acute aortic emergency.
In terms of microscopic anatomy, all blood vessels within the human body are made up of three distinct, concentric cell layers known as “tunics.” The composition of these cell layers greatly vary from one to the next.
- Tunica intimna: This is the inner-most lining of a blood vessel. This layer is mainly comprised of endothelial cells that control the migration of certain molecules and blood cells between the intravascular and extravascular spaces.
- Tunica media: This middle layer is the thickest of the three. Its thickness is proportional to the caliber of the vessel. It’s comprised of elastic tissue (elastin) and smooth muscle. Elastin is an extremely elastic protein that is the aorta’s main load-bearing compound.
- Tunica adventitia: Lastly, the outer layer is a thin coating of connective tissue. The tunica adventitia is fairly confluent with the connective tissue of the vessel’s surrounding environment.
DEFINITIONS & PATHOPHYSIOLOGY
When talking about an aortic catastrophe, in most cases we’re referring to an aortic aneurysm that has dissected. In the most dramatic of scenarios, the aorta blows open and spills a patient’s entire circulating blood volume in a matter just seconds. This worst-case scenario results in sudden death. However, in most other instances an aneurysm tears and begins to leak. This more common scenario results in patients who are symptomatic in some regard.
The distinction between aortic aneurysm and aortic dissection is an important one. Aneurysms of the aorta and any other artery for that matter, are characterized by a demarcated segment of the vessel that has increased in diameter by 1.5 times when compared to the expected caliber of the vessel.1 The process by which an aneurysm forms is highly dependent upon the patient it affects and its location on the aorta. Many people with aortic aneurysms live years without knowing of the condition or suffering any complications from it.
The actual causative agents that lead to the development of an aneurysm are believed to be multifactorial, but aren’t completely understood. There are molecular and mechanical components that cause most aneurysms. As the aorta is subjected to noxious insults such as chronic hypertension and smoking, inflammation occurs within the wall that leads to degradation of elastin and necrosis of smooth muscle cells.2,3
In the portion of the aorta distal to the left subclavian artery, these inflammatory changes lead to development of atherosclerotic plaques, not unlike coronary artery disease (CAD). However, it’s believed the presence of this atherosclerosis within an aneurysm is more associative than causative, as both result from inflammation within the vessel wall. In the ascending aorta, atherosclerosis is rarely seen most likely due to differences in the cellular makeup in this portion of the TA.4
These inflammatory changes slowly begin to degrade the wall of the vessel, causing it to bulge over time. As the bulge continues to grow, it gives way to the aneurysm. Once this degradation of the aneurysm’s wall reaches critical mass, the constant pounding from ventricular contraction ultimately leads to the tearing that causes dissection.
For a thoracic aortic aneurysm (TAA), the etiology varies based on the location of the aneurysm’s inception. For aneurysms proximal to the descending aorta, the causes are heavily associated with alterations or congenital/ genetic abnormalities in the tissue of that portion of the aorta (bicuspid aortic valve, aortic coarctation, surgical aortic valve intervention). Due to the larger proportion of elastin that the TA has, TAAs are typically very silent in their development. Rarely do patients present with complaints associated with TAA development. Most TAAs grow quite slowly (< 0.5 cm/year), with aneurysms proximal to the left subclavian artery growing as slow as 0.1 cm/year.5
In contrast, abdominal aortic aneurisms (AAAs) tend to progress at a faster rate: 0.2 to < 1 cm per year. Similar to TAAs, as AAAs grow larger, their rate of growth also increases.6 AAAs are also generally asymptomatic; however, they’re more commonly associated with symptoms not caused by a dissection (abdominal pain).7
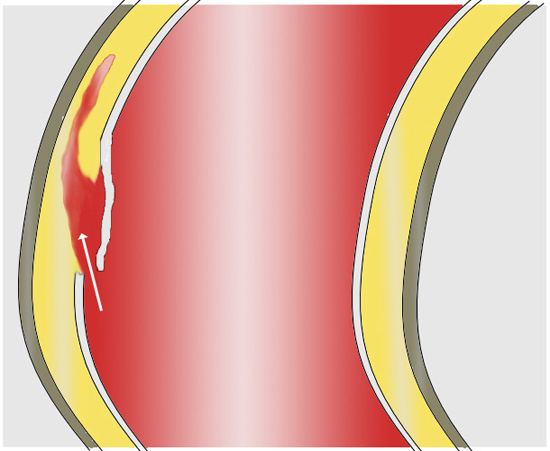
Aortic dissections develop via a small tear in the aorta’s innermost layer. Wikimedia Commons/ J. Heuser
Aortic dissection is the acute tearing of the tunica intima leading to hemorrhage within the medial tunic. The current school of thought regarding the cause of the tear focuses on the presence of hypertension in consort with a chronic aneurysm. There’s an especially high risk of dissection in patients with aneurysm if they develop an acute hypertensive crisis.
There are other non-aneurysm causes of aortic dissection such as acute hypertensive crisis following cocaine abuse and trauma. Both of these conditions lead to a shearing of the aorta and likely will be fatal within moments of their occurrence.
Whatever the cause of the tear, with every beat of the heart, additional blood is forced between the tunics. As blood is forced into this space, the layers of the vessel begin to separate from each other—hence the term “dissection.” The area created by blood filling the space between layers is called the “false lumen.” The false lumen can propagate in either direction away from the tear, and can extend into any other artery in consort with the aorta (coronaries, carotids/cerebrals, vertebrals, renals and peripherals to the limbs). The growth of the false lumen is the actual cause of symptoms for patients. Any progression along these other vessels often leads to ischemic conditions of the associated structures such as myocardial ischemia and stroke.
If the dissection propagates proximally and reaches the aortic root, it can obliterate the aortic valve and/or cause pericardial effusion/tamponade.
The most accepted means of classifying an aortic dissection looks at which parts of the aorta are effected, regardless of the site of the tear or aneurysm. Type A aneurysms must involve the aorta proximal to the left subclavian artery, while Type B aneurysms can involve any part of the aorta except the TA proximal to the left subclavian artery. Type As are associated with an increased morbidity and mortality.
INCIDENCE & RISKS
The incidence of aortic aneurysm significantly favors Caucasian males over the age of 64.6,7 While women tend not to develop aortic aneurysms as readily as males, they are more likely to suffer from dissection.8
There are a host of genetic conditions that predispose patients to aortic aneurysms, especially those that effect collagen formation such as Marfan syndrome and Ehlers-Danlos syndrome. An important distinction among the genetic causes is that they almost never involve an elderly patient. Many times, genetic disorders are the cause of aneurysm dissections in patients less than 40 years of age.9
Additionally, there’s a very strong familial component to the condition. Nearly 20% of aneurysm repair patients also have a first-degree relative suffering from the condition.10
Aside from a known genetic disorder, the two largest risks for the development of an aortic aneurism are a history of hypertension and atherosclerosis. Some of the other risk factors include inflammatory disorders, coronary artery bypass grafting, percutaneous coronary intervention, aortic valve replacement/repair, aneurysm of other moderate caliber vessel(s), bicuspid aortic valve, abnormalities in aortic development (i.e., aortic coarctation), high-intensity weightlifting, and most notably smoking.
For providers trying to discern aortic disease from another condition, there are a host of other risk factors that may play a role in the development of an aneurysm and its subsequent dissection. Unfortunately, these additional risks are shared with numerous other cardiovascular conditions such as coronary artery disease, myocardial infarction (MI), heart failure, etc.
Regardless of the processes leading to the development of an aneurysm, rarely will a prehospital provider encounter patients with an unknown and asymptomatic aneurysm, and be able to diagnose the condition with any high degree of certainty. This being said, the ability to rapidly assess for and rule-in dissection is a whole other matter entirely. Recognition of aortic dissection is of great importance to these patients and quite plausible in the prehospital setting.
PATIENT PRESENTATION
The most common symptom of aortic dissection is pain. The onset is almost always very abrupt and severe. The pain’s quality is usually described as sharp or tearing, with sharp being more common. The location of the pain can vary wildly depending on the location and involved structures. For isolated dissections of the AAA, abdominal and back pain are very likely. For patients with a TAA dissection, posterior chest pain is usually present with anterior chest pain accompanying Type A dissections. Should there be propagation along any confluent vessels, the pain can radiate to almost anywhere. Should a coronary be involved, symptoms of an MI may be present; if a renal artery dissects, flank pain on the affected side is possible; and if an iliac artery is destroyed, the affected ischemic limb could be painful.
Providers should especially be concerned for dissection if a patient presents with a known history of an aortic aneurysm and the sudden onset of the pain described above. Should these same patients with a known aneurysm and sudden chest/back pain also present with a hypertensive state, they’re quite likely to be suffering from an acute dissection. Providers can’t miss this sequela of symptoms!
Because of the variation in the location of an aneurysm and the mobility of the dissection, symptoms can vary greatly from one patient to the next. For instance, if a dissection involves the left subclavian artery, patients will present with a disparity in blood pressure between arms. If the vertebral arteries are involved, there patient may present with paresthesias due to spinal cord ischemia. It’s difficult to diagnose a dissection on the basis of one of these attributing symptoms alone, however these, in addition to the classic symptoms above, should only help solidify your diagnosis.
TRANSPORT
If left untreated, aortic dissection should be considered a death sentence, especially in cases of large or Type A dissections. Should the tunica adventia rupture as a result of a growing dissection, the patient will spill blood into the affected cavity and exsanguinate in a matter of moments.
The greatest asset these patients can be offered by EMS providers is early recognition, followed by appropriate transport to the correct destination. In many areas, these patient require the expert surgical services only available at academic, tertiary care centers. Should transport to such a facility not be possible, providers should make every effort to transport to a facility with cardiothoracic and vascular surgical services on staff. Patients with known aortic aneurysms will typically be able to tell you which hospital they’re followed at and where they should be transported. Aeromedical utilization may also be plausible in consult with a physician colleague via medical command. Aeromedical should be limited to patients with a high degree of suspicion for dissection and are in an area where transport to the appropriate facility isn’t possible within a reasonable amount of time.
MANAGEMENT
Management of these patients focuses on several hallmark therapies. Ultimately, the goal is to keep the patient alive long enough to get into surgery with as few complications as possible.
Initial management is no different than any other medical or surgical emergency, pay attention to your ABCs (airway, breathing and circulation). If a patient’s mental status warrants intubation, intubate and ventilate. If a patient is hypotensive, judiciously resuscitate them.
Next move on to the acute medical management. First, providers must respect the critical role that analgesia has in this illness.
Dissections in the presence of tachycardia and hypertension are extremely dangerous and prone to sudden death. Pain can lead to both of these factors. Aggressive pain management is key in preventing the exacerbation caused by hypertension and tachycardia.
After pain control is accounted for, providers should move on to very tight hemodynamic control. Blood pressure and heart rate control are of great importance to these patients. The initial goal is to ensure a patient’s systolic blood pressure remains between 100–120 mmHg. In patients who are hypertensive with a heart rate > 60 bpm, the initial therapy should be IV beta-blockers.1 My preference for the prehospital and critical care transport setting is esmolol. Although others such as labetalol are more commonly seen in the inpatient setting, esmolol is probably the strongest choice in our environment because it’s highly titratable and has a very short half-life. Should a patient have an unfavorable reaction to esmolol, the infusion can be turned off and its effects will wear off within minutes. Esmolol is very effective at reducing hypertension that’s secondary to increased heart rate, and thus should only be given for patients with heart rates 80 bpm and above. Patients who will only stand for a minimal reduction in heart rate (70–90 bpm) may only have minimal reductions in blood pressure. Do keep in mind, however, that esmolol is a very strong vesicant and should be given through a well-flowing, moderate-bore IV.
If beta-blockers aren’t indicated (i.e., heart rate is < 60 bpm), contraindicated (e.g., allergy, asthma, etc.), or simply ineffective, a nitroprusside infusion should be the next agent of choice. As a direct peripheral vasodilator, nitroprusside is quite effective at quickly decreasing the blood pressure while maintaining the tone and integrity of the aorta. Other direct vasodilators such as hydralazine shouldn’t be administered to these patients as they can decrease the tone of the vessel to the point that it’s more susceptible to increased stress and further tearing.
Any of these treatments in the acute phase of dissection should be via IV infusion to closely monitor their efficacy and titrate as needed.
CONCLUSION

Because of the aorta’s size and involvement in nearly every organ system, dissections can have catastrophic consequences in nearly every part of the body. Wikimedia Commons/ Edoarado
Patients suffering from aortic dissection are indeed at risk of catastrophic outcomes. As dissections progress, patients can have devastating long-term consequences such as renal failure, paralysis, cerebrovascular accident or MI. Additionally, dissections have a high likelihood of precipitously deteriorating, killing patients in a matter of seconds.
Early recognition of this condition is paramount to survival. These patients are often surgical emergencies and require specialized services available at only certain facilities. Rapid transport to the correct facility will greatly ensure the best chance and survival. Lastly, very targeted treatments and tight regulation of hemodynamics by prehospital and critical care providers will greatly increase the chance that these patients make it to the operating room for definitive surgical recovery
REFERENCES
1. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation.2010;121(13):e266–e369.
2. Campa J, Greenhalgh R, Powell J. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987;65(1–2):13–21.
3. Eagleton M. Inflammation in abdominal aortic aneurysms: Cellular infiltrate and cytokine profiles. Vascular. 2012;20(5):278–283.
4. Cheung C, Bernardo AS, Trotter MW, et al. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol.2012;30(2):165–173.
5. Woo YJ, Mohler ER III. (Oct. 27, 2015.) Epidemiology, risk factors, pathogenesis and natural history of thoracic aortic aneurysm.UpToDate. Retrieved Jan. 19, 2016, from www.uptodate.com/contents/epidemiology-risk-factors-pathogenesis-andnatural-history-of-thoracic-aortic-aneurysm.
6. Mohler ER III. (Oct. 4, 2014.) Epidemiology, risk factors, pathogenesis and natural history of abdominal aortic aneurysm.UpToDate. Retrieved Jan. 19, 2016, from www.uptodate.com/contents/epidemiology-risk-factors-pathogenesis-andnatural-history-of-abdominal-aortic-aneurysm.
7. Rahimi S. (Sept. 28, 2015.) Abdominal aortic aneurysm. Medscape.Retrieved Jan. 19, 2016, from http://emedicine.medscape.com/article/1979501-overview.
8. Dillavou E, Muluk S, Makaroun M. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg.2006;43(2):230–238.
9. Januzzi JL, Isselbacher EM, Fattori R, et al. Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol.2004;43(4):665–669.
10. van Vlijmen-van Keulen CJ, Pals G, Rauwerda JA. Familial abdominal aortic aneurysm: A systematic review of a genetic background. Eur J Vasc Endovasc Surg. 2002;24(2):105–116.
RESOURCES
- Jim J, Thompson R. (Sept. 25, 2015.) Clinical features and diagnosis of abdominal aortic aneurysm. UpToDate. Retrieved Jan. 19, 2016, from www.uptodate.com/contents/clinical-features-and-diagnosis-of-abdominal-aortic-aneurysm.
- Manning WJ, Black JH III. (Dec. 30, 2015.) Clinical manifestations and diagnosis of aortic dissection. UpToDate. Retrieved Jan. 19, 2016, from www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-aortic-dissection.
- Woo YJ, Mohler ER III. (Sept. 24, 2013.) Clinical manifestations and diagnosis of thoracic aortic aneurysm. UpToDate. Retrieved Jan. 19, 2016, from www.uptodate.com/contents/clinicalmanifestations-and-diagnosis-of-thoracic-aortic-aneurysm.
Robert P. Girardeau, BS, NREMT-P, FP-C, is a is a critical care and flight paramedic with Thomas Jefferson University Hospital’s Critical Care Transport and Flight Program, JeffSTAT. He’s also an educator and field provider in the greater Philadelphia area. He can be reached at [email protected].
Content retrieved from: http://www.jems.com/articles/print/volume-41/issue-3/features/how-aortic-aneurysms-become-aortic-catastrophes.html.